
HL Paper 2
Consider the following equilibrium reaction:
2SO2 (g) + O2 (g) 2SO3 (g)
State the equilibrium constant expression, Kc, for the reaction above.
State and explain how the equilibrium would be affected by increasing the volume of the reaction container at a constant temperature.
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.
Determine the value of Kc at 600 °C.
Markscheme
«Kc = » ✓
Square brackets required for the mark.
pressure decrease «due to larger volume» ✓
reaction shifts to side with more moles/molecules «of gas» ✓
reaction shifts left/towards reactants ✓
Award M3 only if M1 OR M2 awarded.
[O2] = 1.25 «mol dm−3» AND [SO3] = 3.50 «mol dm−3» ✓
«Kc ==» 4.36 ✓
Award [2] for correct final answer
Examiners report
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
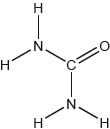
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
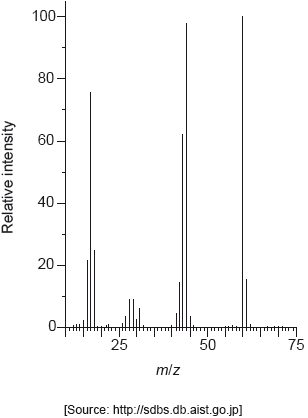
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
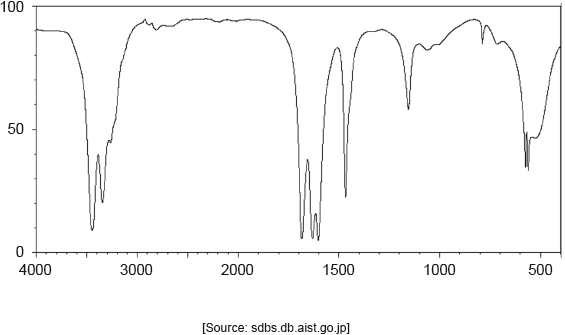
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
Markscheme
molar mass of urea «4 1.01 + 2 14.01 + 12.01 + 16.00» = 60.07 «g mol-1»
«% nitrogen = 100 =» 46.65 «%»
Award [2] for correct final answer.
Award [1 max] for final answer not to two decimal places.
[2 marks]
«cost» increases AND lower N% «means higher cost of transportation per unit of nitrogen»
OR
«cost» increases AND inefficient/too much/about half mass not nitrogen
Accept other reasonable explanations.
Do not accept answers referring to safety/explosions.
[1 mark]

Note: Urea’s structure is more complex than that predicted from VSEPR theory.
[3 marks]
n(KNCO) «= 0.0500 dm3 0.100 mol dm–3» = 5.00 10–3 «mol»
«mass of urea = 5.00 10–3 mol 60.07 g mol–1» = 0.300 «g»
Award [2] for correct final answer.
[2 marks]
[1 mark]
«Kc» decreases AND reaction is exothermic
OR
«Kc» decreases AND ΔH is negative
OR
«Kc» decreases AND reverse/endothermic reaction is favoured
[1 mark]
ln K « = » = –20
«Kc =» 2 10–9
OR
1.69 10–9
OR
10–9
Accept range of 20-20.2 for M1.
Award [2] for correct final answer.
[2 marks]
Any one of:
urea has greater molar mass
urea has greater electron density/greater London/dispersion
urea has more hydrogen bonding
urea is more polar/has greater dipole moment
Accept “urea has larger size/greater van der Waals forces”.
Do not accept “urea has greater intermolecular forces/IMF”.
[1 mark]
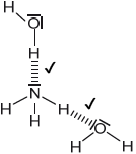
Award [1] for each correct interaction.
If lone pairs are shown on N or O, then the lone pair on N or one of the lone pairs on O MUST be involved in the H-bond.
Penalize solid line to represent H-bonding only once.
[2 marks]
2(H2N)2CO(s) + 3O2(g) → 4H2O(l) + 2CO2(g) + 2N2(g)
correct coefficients on LHS
correct coefficients on RHS
Accept (H2N)2CO(s) + O2(g) → 2H2O(l) + CO2(g) + N2(g).
Accept any correct ratio.
[2 marks]
«V = 22700 cm3 mol–1 =» 227 «cm3»
[1 mark]
lone/non-bonding electron pairs «on nitrogen/oxygen/ligand» given to/shared with metal ion
co-ordinate/dative/covalent bonds
[2 marks]
lone pairs on nitrogen atoms can be donated to/shared with C–N bond
OR
C–N bond partial double bond character
OR
delocalization «of electrons occurs across molecule»
OR
slight positive charge on C due to C=O polarity reduces C–N bond length
[1 mark]
60: CON2H4+
44: CONH2+
Accept “molecular ion”.
[2 marks]
3450 cm–1: N–H
1700 cm–1: C=O
Do not accept “O–H” for 3450 cm–1.
[2 marks]
1
[2 marks]
singlet
Accept “no splitting”.
[1 mark]
acts as internal standard
OR
acts as reference point
one strong signal
OR
12 H atoms in same environment
OR
signal is well away from other absorptions
Accept “inert” or “readily removed” or “non-toxic” for M1.
[2 marks]
Examiners report
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Markscheme
Accept any diagram with each P joined to the other three.
Accept any combination of dots, crosses and lines.
P4 (s) + 6Cl2 (g) → 4PCl3 (l) ✔
Electron domain geometry: tetrahedral ✔
Molecular geometry: trigonal pyramidal ✔
Bond angle: 100«°» ✔
Accept any value or range within the range 91−108«°» for M3.
PCl5 is non-polar:
symmetrical
OR
dipoles cancel ✔
PCl4F is polar:
P–Cl has a different bond polarity than P–F ✔
non-symmetrical «dipoles»
OR
dipoles do not cancel ✔
Accept F more electronegative than/different electronegativity to Cl for M2.
«−398.9 kJ mol−1 − (−306.4 kJ mol−1) =» −92.5 «kJ mol−1» ✔
«ΔS = 364.5 J K–1 mol–1 – (311.7 J K–1 mol–1 + 223.0 J K–1 mol–1)=» –170.2 «J K–1 mol–1» ✔
«ΔS =» –0.1702 «kJ mol–1 K–1»
OR
298 «K» ✔
«ΔG = –92.5 kJ mol–1 – (298 K × –0.1702 kJ mol–1 K–1) =» –41.8 «kJ mol–1» ✔
Award [2] for correct final answer.
If –87.6 and -150.5 are used then –42.8.
«ΔG = –41.8 kJ mol–1 = × 298 K × lnK»
OR
«ΔG = –41800 J mol–1 = –8.31 J mol–1 K–1 × 298 K × lnK»
«lnK = =» 16.9 ✔
«K = e16.9 =» 2.19 × 107 ✔
Award [2] for correct final answer.
Accept range of 1.80 × 106–2.60 × 107.
If –43.5 is used then 4.25 × 107.
«Kc =» ✔
«shifts» left/towards reactants AND «forward reaction is» exothermic/ΔH is negative ✔
Examiners report
A mixture of 1.00 mol SO2(g), 2.00 mol O2(g) and 1.00 mol SO3(g) is placed in a 1.00 dm3 container and allowed to reach equilibrium.
2SO2(g) + O2(g) 2SO3(g)
Nitrogen oxide is in equilibrium with dinitrogen dioxide.
2NO(g) N2O2(g) ΔHΘ < 0
Deduce, giving a reason, the effect of increasing the temperature on the concentration of N2O2.
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
The rate constant for a reaction doubles when the temperature is increased from 25.0 °C to 35 °C.
Calculate the activation energy, Ea, in kJ mol−1 for the reaction using section 1 and 2 of the data booklet.
Markscheme
[N2O2] decreases AND exothermic «thus reverse reaction favoured»
Accept “product” for [N2O2].
Do not accept just “reverse reaction favoured/shift to left” for “[N2O2] decreases”.
[1 mark]
ALTERNATIVE 1:
«from equilibrium, step 1»
OR
[N2O2] = Kc[NO]2
«from step 2, rate «= k1[N2O2][O2] = k2K[NO]2[O2]»
rate = k[NO]2[O2]
ALTERNATIVE 2:
«from step 2» rate = k2[N2O2][O2]
«from step 1, rate(1) = k1[NO]2 = k–1[N2O2], [N2O2] = [NO]2»
«rate = k2[NO]2[O2]»
rate = k[NO]2[O2]
Award [2] for correct rate expression.
[2 marks]
«»
T2 = «273 + 35 =» 308 K AND T1 = «273 + 25 =» 298 K
Ea = 52.9 «kJ mol–1»
Award [2] for correct final answer.
[2 marks]
Examiners report
A student performs a titration to determine the concentration of ethanoic acid, , in vinegar using potassium hydroxide.
The pH curve for the reaction is given.
Write a balanced equation for the reaction.
Identify the major species, other than water and potassium ions, at these points.
State a suitable indicator for this titration. Use section 22 of the data booklet
Suggest, giving a reason, which point on the curve is considered a buffer region.
State the expression for ethanoic acid.
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
In a titration, of vinegar required of potassium hydroxide to reach the end-point.
Calculate the concentration of ethanoic acid in the vinegar.
Potassium hydroxide solutions can react with carbon dioxide from the air. The solution was made one day prior to using it in the titration.
State the type of error that would result from the student’s approach.
Potassium hydroxide solutions can react with carbon dioxide from the air. The solution was made one day prior to using it in the titration.
Predict, giving a reason, the effect of this error on the calculated concentration of ethanoic acid in 5(e).
Markscheme
✔
Accept the ionic equation.
B: AND ✔
C: ✔
Accept names.
Accept for
phenolphthalein ✔
Accept “phenol red” or “bromothymol blue”.
B AND the region where small additions «of the base/ » result in little or no
change in
OR
B AND the flattest region of the curve «at intermediate /before equivalence
point »
OR
B AND half the volume needed to reach equivalence point
OR
B AND similar amounts of weak acid//ethanoic acid AND conjugate base//ethanoate ✔
Accept instead of .
✔
Accept answers between .
✔
✔
Award [2] for correct final answer.
systematic «error» ✔
would be higher ✔
actual is lower «than the value in calculation»
OR
larger volume of «solution» needed to neutralize the acid ✔
Accept partially neutralised by from air.
Examiners report
Most candidates could write a balanced neutralization equation.
Identifying species present at various points along a pH titration curve was one of the most poorly answered questions in the exam. Very few candidates realized there were two major species at point B even when they were able in general to realize that B was a buffer zone.
Almost all candidates could identify a suitable indicator to use in a titration of a weak acid with a strong base.
Most students could identify a buffer zone region in a titration but very few (50%) could coherently explain why.
Poorly answered with only 50% correctly writing a Ka expression. The major error was in candidates trying to calculate a Ka rather than write an expression for it.
Like with other calculations in this exam, the majority of candidates could correctly determine a concentration from titration data.
80% of candidates could identify the method used as a systematic error, with some stating human or random error.
Most candidates identified that the systematic error would result in the concentration of the alkali being lowered but then failed to propagate this through to the effect on the concentration of the acid.
Many reactions are in a state of equilibrium.
The following reaction was allowed to reach equilibrium at 761 K.
H2 (g) + I2 (g) 2HI (g) ΔHθ < 0
The pH of 0.010 mol dm–3 carbonic acid, H2CO3 (aq), is 4.17 at 25 °C.
H2CO3 (aq) + H2O (l) HCO3– (aq) + H3O+ (aq).
State the equilibrium constant expression, Kc , for this reaction.
The following equilibrium concentrations in mol dm–3 were obtained at 761 K.
Calculate the value of the equilibrium constant at 761 K.
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
Calculate [H3O+] in the solution and the dissociation constant, Ka , of the acid at 25 °C.
Calculate Kb for HCO3– acting as a base.
Markscheme
Kc =
45.6
ΔGθ = «– RT ln K = – (0.00831 kJ K−1 mol−1 x 761 K x ln 45.6) =» – 24.2 «kJ»
[H3O+] = 6.76 x 10–5 «mol dm–3»
Ka =
4.6 x 10–7
Accept 4.57 x 10–7
Award [3] for correct final answer.
« =» 2.17 x 10–8
OR
« =» 2.19 x 10–8
Examiners report
This reaction is used in the manufacture of sulfuric acid.
2SO2 (g) + O2 (g) 2SO3 (g) Kc = 280 at 1000 K
State why this equilibrium reaction is considered homogeneous.
Predict, giving your reason, the sign of the standard entropy change of the forward reaction.
Calculate the standard Gibbs free energy change, ΔGΘ, in kJ, for this reaction at 1000 K. Use sections 1 and 2 of the data booklet.
Predict, giving your reasons, whether the forward reaction is endothermic or exothermic. Use your answers to (b) and (c).
0.200 mol sulfur dioxide, 0.300 mol oxygen and 0.500 mol sulfur trioxide were mixed in a 1.00 dm3 flask at 1000 K.
Predict the direction of the reaction showing your working.
Markscheme
all «species» are in same phase ✔
Accept “all species are in same state”.
Accept “all species are gases”.
negative AND fewer moles/molecules «of gas» in the products ✔
ΔGΘ =«–RT ln Kc =» –8.31 J K–1 mol–1 × 1000 K × ln 280
OR
ΔGΘ = – 4.7 × 104 «J» ✔
«ΔGΘ =» – 47 «kJ» ✔
Award [2] for correct final answer.
ΔGΘ < 0/spontaneous AND ΔSΘ < 0/unfavourable ✔
exothermic AND ΔHΘ «must be» negative/favourable ✔
«reaction quotient/Q =» ✔
reaction quotient/Q/20.8/answer < Kc/280
OR
mixture needs more product for the number to equal Kc ✔
reaction proceeds to the right/products ✔
Do not award M3 without valid reasoning.
Examiners report
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Now consider the second stage of the reaction.
CO (g) + 2H2 (g) CH3OH (l) ΔH⦵ = –129 kJ
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
CO (g) + 2H2 (g) CH3OH (l)
Consider the first stage of the reaction.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1.
The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product.
Determine the overall equation for the production of methanol.
8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet.
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
State one reason why you would expect the value of ΔH calculated from the values, given in section 12 of data booklet, to differ from your answer to (d)(i).
State the expression for Kc for this stage of the reaction.
State and explain the effect of increasing temperature on the value of Kc.
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
Calculate a value for the entropy change, ΔS⦵, in J K–1 mol–1 at 298 K. Use your answers to (e)(i) and section 1 of the data booklet.
If you did not get answers to (e)(i) use –1 kJ, but this is not the correct answer.
Justify the sign of ΔS with reference to the equation.
Predict, giving a reason, how a change in temperature from 298 K to 273 K would affect the spontaneity of the reaction.
Markscheme
curve higher AND to left of T1 ✔
new/catalysed Ea marked AND to the left of Ea of curve T1 ✔
Do not penalize curve missing a label, not passing exactly through the origin, or crossing x-axis after Ea.
Do not award M1 if curve drawn shows significantly more/less molecules/greater/smaller area under curve than curve 1.
Accept Ea drawn to T1 instead of curve drawn as long as to left of marked Ea.
methanoic acid/HCOOH/CHOOH
OR
methanal/HCHO ✔
Accept “carbon dioxide/CO2”.
CH4(g) + H2O(g) CH3OH(l) + H2(g) ✔
Accept arrow instead of equilibrium sign.
amount of methane = « = » 0.498 «mol» ✔
amount of hydrogen = amount of methane / 0.498 «mol» ✔
volume of hydrogen = «0.498 mol × 22.7 dm3 mol−1 = » 11.3 «dm3» ✔
Award [3] for final correct answer.
Award [2 max] for 11.4 «dm3 due to rounding of mass to 16/moles to 0.5. »
Σbonds broken = 4 × 414 «kJ» + 2 × 463 «kJ» / 2582 «kJ» ✔
Σbonds formed = 1077 «kJ» + 3 × 436 «kJ» / 2385 «kJ» ✔
ΔH «= Σbonds broken − Σbonds formed =( 2582 kJ − 2385 kJ)» = «+»197«kJ» ✔
Award [3] for final correct answer.
Award [2 Max] for final answer of −197 «kJ»
bond energies are average values «not specific to the compound» ✔
✔
Kc increases AND «forward» reaction endothermic ✔
«ΔG⦵ = − RT lnKc»
ΔG⦵ = − 8.31 «J K−1 mol−1» × 298 «K» × ln (1.01) / −24.6 «J mol−1» ✔
= −0.0246 «kJ mol–1» ✔
Award [2] for correct final answer.
Award [1 max] for +0.0246 «kJ mol–1».
«ΔG⦵ = ΔH⦵ − TΔS⦵»
ΔG⦵ = −129 «kJ mol–1» − (298 «K» × ΔS) = −0.0246 «kJ mol–1» ✔
ΔS⦵ = « = » −433 «J K–1 mol–1» ✔
Award [2] for correct final answer.
Award [1 max] for “−0.433 «kJ K–1 mol–1»”.
Award [1 max] for “433” or “+433” «J K–1 mol–1».
Award [2] for −430 «J K–1 mol–1» (result from given values).
«negative as» product is liquid and reactants gases
OR
fewer moles of gas in product ✔
reaction «more» spontaneous/ΔG negative/less positive AND effect of negative entropy decreases/TΔS increases/is less negative/more positive
OR
reaction «more» spontaneous/ΔG negative/less positive AND reaction exothermic «so Kc increases » ✔
Award mark if correct calculation shown.
Examiners report
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR
State the type of hybridization shown by the central carbon atom in molecule B.
State the number of sigma () and pi () bonds around the central carbon atom in molecule B.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
Calculate the standard Gibbs free energy change, , in , for the reaction (A to B) at . Use sections 1 and 2 of the data booklet.
Propanone can be synthesized in two steps from propene. Suggest the synthetic route including all the necessary reactants and steps.
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
Markscheme
Electron domain geometry: tetrahedral ✔
Molecular geometry: bent/V-shaped ✔
✔
-bonds:
AND
-bonds: ✔
B AND absorption/
OR
B AND absence of ✔
Accept any value between .
Accept any two isomers except for propanone and propen-2-ol:
✔✔
Penalize missing hydrogens in displayed structural formulas once only.
AND is greater than /large ✔
✔
/water «and » ✔
/propan-2-ol ✔
/«potassium» dichromate(VI) AND
OR
/«acidified potassium» manganate(VII) ✔
Accept .
primary carbocation «intermediate forms»
OR
minor product «of the water addition would be» propan-1-ol
OR
anti-Markovnikov addition of water ✔
primary alcohol/propan-1-ol oxidizes to an aldehyde/propanal ✔
Examiners report
The majority of students got at least one of electron domain geometry or molecular geometry correct.
The vast majority of students could identify the hybridization around a central carbon atom.
The vast majority of students could identify BOTH sigma and pi bonds in a molecule.
Many candidates identified B having C = O and a peak at 1750.
A surprising number of candidates drew propanone here as an option, either failing to read the question or perhaps finding the structural formulae provided difficult to understand.
Most candidates identified B, the product, as being in greater concentration at equilibrium however some lost the mark because they did not include a reason.
Most candidates could apply the formula for Gibbs free energy change, ΔGΘ, correctly however some did not get the units correct.
The mean mark was ⅔ for the required synthetic route. Some candidates failed to identify water as a reagent in the hydration reaction, or note that dichromate ion oxidation requires acidic conditions. This was also the question with most No Response.
This question regarding the formation of a minor product was not well answered. Many candidates struggled to explain the formation of propan-1-ol and to then oxidize it to propanal.